Answers
Answer:
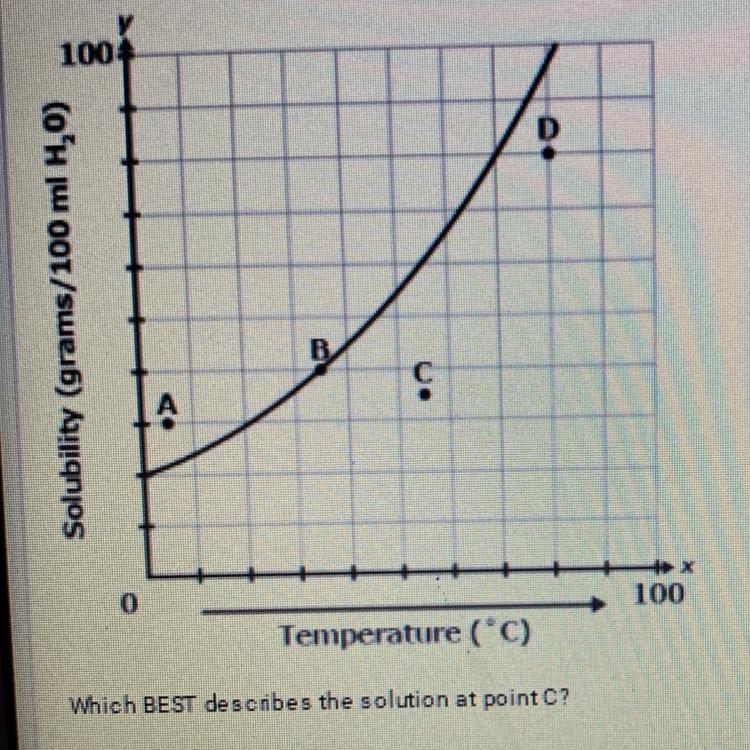
No Its B Unsaturated becuase the line is slanted
Explanation:
Related Questions
The density of ice is 0.92 g/cm3 and the density of rubbing alcohol is 0.80 g/cm3. If an ice cube is placed in a container of rubbing alcohol, the ice cube will
Question 7 options:
a) float in the rubbing alcohol with none of the ice cube submerged.
b) sink to the bottom of the container.
c) move up and down in the rubbing alcohol.
d) float in the alcohol so that most of the ice cube is below the surface of the liquid.
Answers
Answer:
Option B. Sink to the bottom of the container.
Explanation:
From the question given above, the following data were obtained:
Density of ice = 0.92 g/cm³
Density of rubbing alcohol = 0.80 g/cm³
From the density of both substance given, we can see clearly that the ice has a higher density when compared with the rubbing alcohol.
Thus, If an ice cube is placed in a container of rubbing alcohol, the ice cube will not float rather, it will sink to the bottom of the container because the ice has a higher density than the rubbing alcohol.
Why are the coefficients before a chemical symbol important?F. They show the number of elements in each molecule.G. They indicate the number of molecules of each substance.H. They describe the number of times each molecule reacts.J. They explain the order in which the reaction takes place
Answers
Answer:They indicate the number of molecules of each substance
Explanation:
When we write chemical reaction equations, we usually put a number before the symbol of each reactant.
These coefficient shows the number of molecules of that specie that is involved in the balanced reaction equation for the reaction.
These coefficients are important because they are used in carrying out stoichiometric calculations.
50 POINTS: PLEASE ANSWER AS QUICK AS POSSIBLE!
Question:
After getting their results in, a group of scientists went back and looked at their study. As planned, their experiment consisted of three groups. The first group received a new medicine at a normal dose, the second group received the medicine at half the dose, and the third group received the medicine at twice the dose.
Which of the following best describes what went wrong with the scientists’ study?
Choices:
- an improper experimental procedure
- the lack of a control group
- selection bias
- human error
Answers
Explanation:
The lack of a control group
ultraviolet radiation has a higher frequency than visible light. which type of light wave carries more energy?
a=they carry the same amount of energy
b=visible light
c= ultraviolet radiation
Answers
Answer:
The answer to this question is C; ultraviolet radiation
Explanation:
The the reason for this is because it carries more energy per photon than visible light does. Light travels at a speed of 299,792 kilometers per second (about 186,282 miles per second).
True/False: Units for gas density are grams/L
Answers
Answer:
false
Explanation:
Use the rules of significant figures to report the answer to this math question: (454.3 cL x 17.84 dL )/3.1 L =
Answers
Answer:
2614.423226
Explanation:
Ore deposits contain high amounts of
A) water
B) volcanic magma
C) metals
D) minerals
Answers
what is the identity of the element that produced the unknown emission spectrum
Answers
Answer:
The emitted light can be observed as a series of colored lines with dark spaces in between; this series of colored lines is called a line or atomic spectra.
Explanation:
What do we call the molecule that forms when two atoms of the same element are covalently bonded to each other?
Answers
Answer:
Covalent Compounds and Diatomic Elements
Explanation: The two atoms that are held together by a covalent bond may be atoms of the same element or different elements. When atoms of different elements form covalent bonds, a new substance, called a covalent compound, results. Water is an example of a covalent compound.
Describe how evaporation relates to heat regulation in your body.
Answers
A solution that has a pH of 5
Answers
Answer:
don't you have any options
What is ionization energy?
Answers
Do you think your meal would allow your cells to sufficiently build your cell
membranes? Why or why not?
Answers
Answer:
Cells are the fundamental units of life ' the bricks from which all your. which are built of your cells, will become compromised, and you can. of these new cells from the nutrients you get in your food is one way. Let's take a look inside one of your cells and see what the nutrients really do.
Explanation:
What is the name of this ionic compound (Fr2SO4)
Answers
Answer:
3.5: Ionic Compounds- Formulas and Names
Last updatedAug 25, 2020
3.4: An Atomic-Level Perspective of Elements and Compounds
3.6: Molecular Compounds- Formulas and Names
picture_as_pdf
Readability
Donate
6.9: Binary Ionic Compounds and Their Properties
6.18: Ionic Compounds Containing Polyatomic Ions
Learning Objectives
Derive names for common types of inorganic compounds using a systematic approach
Nomenclature, a collection of rules for naming things, is important in science and in many other situations. This module describes an approach that is used to name simple ionic and molecular compounds, such as NaCl, CaCO3, and N2O4. The simplest of these are binary compounds, those containing only two elements, but we will also consider how to name ionic compounds containing polyatomic ions, and one specific, very important class of compounds known as acids (subsequent chapters in this text will focus on these compounds in great detail). We will limit our attention here to inorganic compounds, compounds that are composed principally of elements other than carbon, and will follow the nomenclature guidelines proposed by IUPAC. The rules for organic compounds, in which carbon is the principle element, will be treated in a later chapter on organic chemistry.
Ionic Compounds
To name an inorganic compound, we need to consider the answers to several questions. First, is the compound ionic or molecular? If the compound is ionic, does the metal form ions of only one type (fixed charge) or more than one type (variable charge)? Are the ions monatomic or polyatomic? If the compound is molecular, does it contain hydrogen? If so, does it also contain oxygen? From the answers we derive, we place the compound in an appropriate category and then name it accordingly.
Compounds Containing Only Monatomic Ions
The name of a binary compound containing monatomic ions consists of the name of the cation (the name of the metal) followed by the name of the anion (the name of the nonmetallic element with its ending replaced by the suffix –ide). Some examples are given in Table 3.5.2 .
Table 3.5.1 : Names of Some Ionic Compounds
NaCl, sodium chloride Na2O, sodium oxide
KBr, potassium bromide CdS, cadmium sulfide
CaI2, calcium iodide Mg3N2, magnesium nitride
CsF, cesium fluoride Ca3P2, calcium phosphide
LiCl, lithium chloride Al4C3, aluminum carbide
Compounds Containing Polyatomic Ions
Compounds containing polyatomic ions are named similarly to those containing only monatomic ions, except there is no need to change to an –ide ending, since the suffix is already present in the name of the anion. Examples are shown in Table 3.5.2 .
Table 3.5.2 : Names of Some Polyatomic Ionic Compounds
KC2H3O2, potassium acetate (NH4)Cl, ammonium chloride
NaHCO3, sodium bicarbonate CaSO4, calcium sulfate
Al2(CO3)3, aluminum carbonate Mg3(PO4)2, magnesium phosphate
Ionic Compounds in Your Cabinets
Ionic Compound Use
NaCl, sodium chloride ordinary table salt
KI, potassium iodide added to “iodized” salt for thyroid health
NaF, sodium fluoride ingredient in toothpaste
NaHCO3, sodium bicarbonate baking soda; used in cooking (and as antacid)
Na2CO3, sodium carbonate washing soda; used in cleaning agents
NaOCl, sodium hypochlorite active ingredient in household bleach
CaCO3 calcium carbonate ingredient in antacids
Mg(OH)2, magnesium hydroxide ingredient in antacids
Al(OH)3, aluminum hydroxide ingredient in antacids
NaOH, sodium hydroxide lye; used as drain cleaner
K3PO4, potassium phosphate food additive (many purposes)
MgSO4, magnesium sulfate added to purified water
Na2HPO4, sodium hydrogen phosphate anti-caking agent; used in powdered products
Na2SO3, sodium sulfite preservative
Table 3.5.3 : Names of Some Transition Metal Ionic Compounds
Transition Metal Ionic Compound Name
FeCl3 iron(III) chloride
Hg2O mercury(I) oxide
HgO mercury(II) oxide
Cu3(PO4)2 copper(II) phosphate
Naming Ionic Compounds
Name the following ionic compounds, which contain a metal that can have more than one ionic charge:
Fe2S3
CuSe
GaN
CrCl3
Ti2(SO4)3
Solution
The anions in these compounds have a fixed negative charge (S2−, Se2− , N3−, Cl−, and SO2−4 ), and the compounds must be neutral. Because the total number of positive charges in each compound must equal the total number of negative charges, the positive ions must be Fe3+, Cu2+, Ga3+, Cr4+, and Ti3+. These charges are used in the names of the metal ions:
iron(III) sulfide
copper(II) selenide
gallium(III) nitride
chromium(III) chloride
titanium(III) sulfate
Exercise 3.5.1
Write the formulas of the following ionic compounds:
(a) chromium(III) phosphide
(b) mercury(II) sulfide
(c) manganese(II) phosphate
(d) copper(I) oxide
(e) chromium(VI) fluoride
Answer
(a) CrP; (b) HgS; (c) Mn3(PO4)2; (d) Cu2O; (e) CrF6
Explanation:
The given compound is francium sulfate.
Explanation:
While naming ionic compounds :
First, write the name of cation Then after that name of the anion.Given
The molecule formula of a compound[tex]Fr_2SO_4[/tex]:
To find:
The name of the given compound formula
Solution:
Cation in formula = [tex]Fr^+[/tex] = Francium cation
Anion in the formula= [tex]SO_4^{-2}[/tex] = Sulfate anion
So, the name of the given compound is francium sulfate.
Learn more about the naming of compounds here:
brainly.com/question/863443?referrer=searchResults
brainly.com/question/8968140?referrer=searchResults
WILL MARK BRAINLIEST!!!! HELPPPP
what is the difference between simple distillation and fractional distillation
Answers
Answer:
A single distillation cycle is enough to separate the mixture. ... Simple distillation is the method used to separate substances in mixtures with significantly different boiling points, while fractional distillation is used for mixtures containing chemicals with boiling points close to each other.
Explanation:
The modern synthesis combined the concepts of _______ and evolution.
Answers
Answer:
genetics
hope this helps :)
Explanation:
can you help me with percent abundance??
Answers
Percent abundance is the percentage of all the different isotopes of an element and is used to calculate the average atomic mass. For example 99.98% of all Hydrogen in the universe is [tex]^1H[/tex] meaning hydrogen with 1 proton and 0 neutrons while the remaining 0.02% is [tex]^2H[/tex] meaning that [tex]^1H[/tex] is the most abundant by percent.
The maximum number of electrons that can fill the second energy level
Answers
Answer:
8
Explanation:
Volcanic belts form along
a.
islands in the Pacific Ocean.
b.
North American mountain ranges.
c.
the boundaries of Earth’s plates.
d.
the coast of Antarctica.
Please select the best answer from the choices provided
Answers
what property of matter describe how matter reacts with other matter ?
a.flammable
b.color
c.shape
d.volume
Answers
Answer:
Flammability
Explanation:
Flammability is a chemical property. So, this means that it must react with a substance.
Color, shape, and volume are physical traits. This means that no other matter needs to be included in order for this trait to be so.
The diagram shows a stage in meiosis.
Which stage of meiosis is pictured?
metaphase I
anaphase I
metaphase II
anaphase II
Answers
Answer:
It's not A or D
Explanation:
I was looking all over for answers and I saw A and D, so I used A. It was wrong, so I retook it and used D. Wrong. Your answer is either B or C. I am sorry I couldn't help more.
Answer:
it is b same question was on my quiz
Explanation:
Michelle learns in science class that simple machines such as an inclined plane can change the amount of force needed to lift heavy objects. She decides to test this with an experiment.
Michelle chooses a 10 kg weight. She sets up a ramp made of smooth metal that makes an angle θ with the floor. She attaches a spring scale to the weight and the top of the ramp in order to hold the weight in place. She records the force from the spring scale, then changes θ and records it again. She repeats this several times.
In this experiment, what is the dependent variable?
A.
the mass of the weight
B.
the angle θ between the ramp and the ground
C.
the amount of force on the spring scale
D.
the material the ramp is made of
Be fast please
Answers
If an object has a mass of 180 g and a density of 6 g/cm^3, what is the volume of the object?
Answers
Answer:
The answer is 30 cm³Explanation:
The volume of a substance when given the density and mass can be found by using the formula
[tex]volume = \frac{mass}{density} \\[/tex]
From the question
mass = 180 g
density = 6 g/cm³
We have
[tex]volume = \frac{180}{6} \\ [/tex]
We have the final answer as
30 cm³Hope this helps you
1. The ability to dissolve in water and to conduct
electricity are examples of
a. physical properties.
b. chemical changes.
c. chemical properties.
d. chemical bonding.
Answers
Answer:
Physical
Explanation:
Because thats the answer
Is this actually right
Answers
Answer:
what?????????i don't see a question
Answer:
Yes
Explanation:
I am tryna make sure of my answer is this correct
Answers
Answer:
great job!!
Explanation:
ALL IS CORRECT :)
3. In an exercise to teach students how to use and analytical balance, the instructor
gives a student a quarter which has been pre-weighed as 5.6026 g. The weight that the
student obtains for the same quarter is 5.6013 g. What is the percent error in the
students reading?
Answers
Answer:
The percent error is 0.023%.
Explanation:
Pre-weighed of a quarter is 5.6026 g.
The weight that the student obtains for the same quarter is 5.6013 g.
We need to find the percent error in the students reading. It is given by the formula as follows :
[tex]\%=\dfrac{|\text{original value-calculated value}|}{\text{original value}}\times 100[/tex]
Putting all the values, we get :
[tex]\%=\dfrac{5.6026 -5.6013 }{5.6026 }\times 100\\\\\%=0.023\%[/tex]
So, the percent error is 0.023%.
The speed of light in plastic is 2.0 x 108 m/s. Calculate the index of refraction for
plastic.
Answers
Answer:
n=c/v.
1.5=3×10^8/v
v=3×10^8/1.5
v=2×10^8 m/s
Hope this helps :)
The index of refraction for plastic, when speed of light in plastic is 1.5.
How do we calculate refraction index?Refraction index of any substance will be calculated by using the below equation:
n = c/v, where
c = speed of light in vaccum = 3×10⁸ m/s
v = speed of light in plastic = 2×10⁸ m/sec
On putting values in the above equation, we get
n = 3×10⁸ / 2×10⁸ = 1.5
Hence the refractive index of plastic is 1.5.
To know more about refractive index, visit the below link:
https://brainly.com/question/83184
#SPJ2
8. Which word best describes the element in box number 2?
a а Brittle
b Gas
С Semiconductor
d. Shiny
Answers
Answer:
gas
Explanation:
i think it might be gas
how many atoms can you fit on the head of a pin
Answers
Answer:
According to google, "About five million million hydrogen atoms could fit. Some factors would affect that number like the area of the head and the size of atoms (as well as attractions between atoms). Some atoms are larger than others." Is this accurate? I'm not sure. Good luck! :)