Answers
Answer:
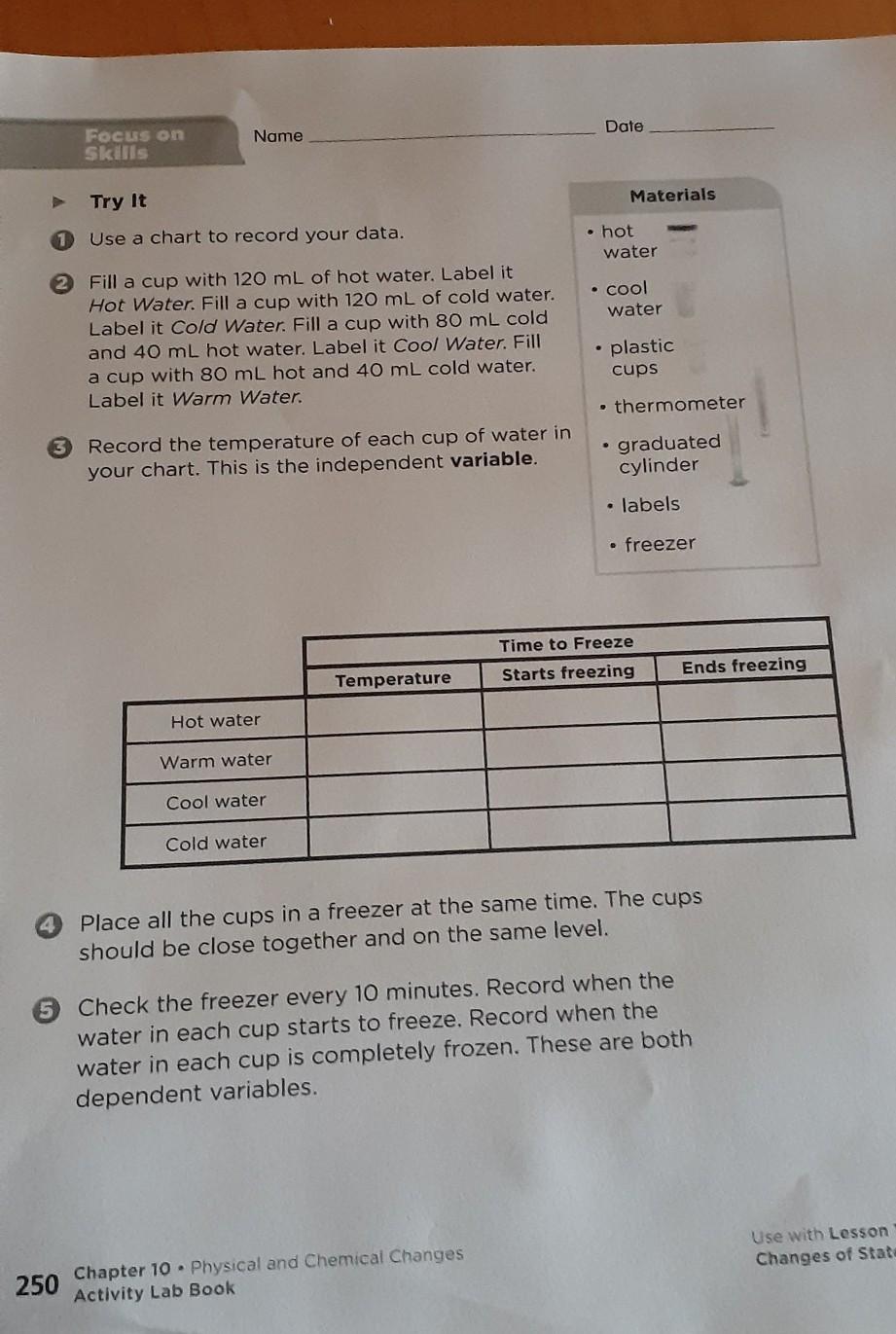
I think you have to freeze the water itself then take the temp with a thermometer ️of each one then record how long it takes each one to start freezing then how long it takes for it to melt
Explanation:
sorry if I'm wrong but you have to do the experiment yourself then fill it out
Related Questions
In cells, the role of the mitochondrion is to:
Question 8 options:
keep the DNA enclosed
allow nutrients to come into the cell
make proteins
produce energy
Answers
Answer:
D produce energy
Explanation:
Mitochondria are membrane-bound cell organelles (mitochondrion, singular) that generate most of the chemical energy needed to power the cell's biochemical reactions.
Answer:produce energy
Explanation:
Pls someone help rn!! Pls pls
Answers
Answer:
A. Mass = 61kg, weight on moon = 97.8N
Explanation:
To calculate the weight (force) from Mass of a body, the following formula is used:
W = mg
Where;
W = weight (Newtons)
m = mass (kg)
g = acceleration due to gravity (9.8m/s²)
According to this question, a scale shows that I weigh 600N on Earth, the mass on Earth can be calculated thus:
Using W = mg
m = W/g
m = 600/9.8
m = 61.2
m = 61kg
However, this mass on Earth will weigh differently on the Moon because gravity in the moon is 1.6 N/kg. The weight of the 61kg on the Moon is:
W(moon) = mass × gravity
W = 61 × 1.6
W = 97.6 N
Therefore, Mass = 61kg, weight on moon = 97.8N
What is the molarity of a solution made by dissolving 1.25 mol of HCl in enough
water to make 625 mL of solution?
A) 0.073 M
B) 2.00 M
OC) 28.5 M
D) 500 M
Answers
Answer:
Explanation:
C
2.00 M is the molarity of a solution made by dissolving 1.25 mol of HCl in enough water to make 625 mL of solution.
What are moles?A mole is defined as 6.02214076 × of some chemical unit, be it atoms, molecules, ions, or others. The mole is a convenient unit to use because of the great number of atoms, molecules, or others in any substance.
[tex]Molality = \frac{Moles \;solute}{Volume \;of \;solution \;in \;litre}[/tex]
[tex]Molality = \frac{1.25}{0.625}[/tex]
Molality = 2.00 M
Learn more about moles here:
brainly.com/question/8455949
#SPJ2
A 497.9-gallon steel storage tank contains 91.7kg of methane. If the temperature is 23.7˚C what is the pressure inside the tank?
Answers
Answer:
74.08 atm
Explanation:
We'll begin by converting 91.7 kg to grams (g). This can be obtained as follow:
1 kg = 1000 g
Therefore,
91.7 kg = 91.7 kg × 1000 g / 1 kg
91.7 kg = 91700 g
Next, we shall determine the number of mole present in 91700 g of methane, CH₄. This can be obtained as follow:
Mass of CH₄ = 91700 g
Molar mass of CH₄ = 12 + (4"1)
= 12 + 4
= 16 g/mol
Mole of CH₄ =?
Mole = mass /Molar mass
Mole of CH₄ = 91700 / 16
Mole of CH₄ = 5731.25 moles
Next, we shall convert 497.9 gallon to litres (L). This can be obtained as follow:
1 gallon = 3.785 L
Therefore,
497.9 gallon = 497.9 × 3.785
497.9 gallon = 1884.55 L
Next, we shall convert 23.7 ˚C to Kelvin temperature. This can be obtained as follow:
T(K) = T(˚C) + 273
T(˚C) = 23.7 ˚C
T(K) = 23.7 °C + 273
T(K) = 296.7 K
Finally, we shall determine the pressure in the tank as follow:
Number of mole (n) = 5731.25
Volume = 1884.55 L
Temperature (T) = 296.7 K
Gas constant (R) = 0.0821 atm.L/Kmol
Pressure (P) =?
PV = nRT
P × 1884.55 = 5731.25 × 0.0821 × 296.7
P × 1884.55 = 139607.92
Divide both side by 1884.55
P = 139607.92 / 1884.55
P = 74.08 atm
Therefore the pressure in the tank is 74.08 atm
One component of smog is nitrogen
monoxide, NO. A car produces about 8 g of
this gas per day. What is the volume at STP?
Answers
Answer:
V = 5.97 L
Explanation:
Hello!
In this case, according to the ideal gas law:
[tex]PV=nRT[/tex]
We would need to solve for V as we know the temperature and pressure as the gas it at STP conditions (273 K and 1 atm respectively):
[tex]V=\frac{nRT}{P}[/tex]
Next, we compute the moles in 8 g of NO, given its molar mass of 30.01 g/mol:
[tex]n=\frac{8g}{30.01g/mol}=0.267mol[/tex]
Therefore, we obtain the following volume:
[tex]V=\frac{0.267mol*0.08206\frac{atm*L}{mol*K}*273K}{1atm}\\\\V=5.97 L[/tex]
Best regards!
The extraction of iron from ore is represented by the chemical reaction equation.
Fe2O3 + 3CO → 2Fe + 3CO2
Calculate the mass of carbon (II) oxide required for the reduction of 40 kg of iron (III) oxide
Answers
Answer:
21 Kg of CO.
Explanation:
The balanced equation for the reaction is given below:
Fe₂O₃ + 3CO —> 2Fe + 3CO₂
Next, we shall determine the masses of Fe₂O₃ and CO that reacted from the balanced equation. This can be obtained as illustrated below:
Molar mass of Fe₂O₃ = (56×2) + (16×3)
= 112 + 48
= 160 g/mol
Mass of Fe₂O₃ from the balanced equation = 1 × 160 = 160 g
Molar mass of CO = 12 + 16 = 28 g/mol
Mass of CO from the balanced equation = 3 × 28 = 84 g
SUMMARY:
From the balanced equation above,
160 g Fe₂O₃ required 84 g of CO.
Finally, we shall determine the mass of of CO required for the reduction of 40 kg of Fe₂O₃. This can be obtained as follow:
From the balanced equation above,
160 g Fe₂O₃ required 84 g of CO.
Therefore, 40 Kg of Fe₂O₃ will require = (40 kg × 84 g) / 160 g = 21 Kg of CO.
Thus, 21 Kg of CO is required for the reaction.
What is the mass of 0.063x10^-4 moles of aluminum sulphate ?
Answers
Answer:
The mass of 0.063*10⁻⁴ moles of aluminum sulphate is 2.15*10⁻³ grams.
Explanation:
Aluminum sulfate Al₂(SO₄)₃ has a molar mass of 342.15 g/mol.
Molar mass is the amount of mass that a substance contains in one mole of a substance, which can be an element or a compound.
So in this case you can apply the following rule of three: if 342.15 grams are present in 1 mole of aluminum sulfate, how much mass is present in 0.063*10⁻⁴ moles of the compound?
[tex]mass of aluminum sulphate=\frac{0.063*10^{-4}moles*342.15 grams }{1 mole}[/tex]
mass of aluminum sulphate= 2.15*10⁻³ grams
The mass of 0.063*10⁻⁴ moles of aluminum sulphate is 2.15*10⁻³ grams.
What is the molar mass of V(CO3)2?
Answers
Just like the other person said ^, hope its right
Fill in the blanks. 3NH3
Answers
Answer:
3, 9, 3
Explanation:
The coefficient of 3 tells us that there are three molecules (the chemical unit of NH3). Each molecule of ammonia (NH3) is made up of 1 atom of nitrogen bonded to 3 atoms of hydrogen.
Since there are three molecules, we have three times the amount of atoms there are in one molecule.
3 x 1 = 3 nitrogen
3 x 3 = 9 hydrogen
(b) The student collects the H2(g) produced by the reaction and measures its volume over water at 298 K after carefully
equalizing the water levels inside and outside the gas-collection tube, as shown in the diagram below. The volume is
measured to be 45.6 mL. The atmospheric pressure in the lab is measured as 765 torr, and the equilibrium vapor pressure
of water at 298 K is 24 torr,
45
46
Gas
Water
Calculate the following.
() The number of moles of h2 produced in the reaction
Answers
Answer:
The pressure of H₂(g) = 741 torr
Explanation:
Given that:
The atmospheric pressure measured in the lab = 765 torr
The vapor pressure of water = 24 torr
By applying Dalton's Law of Partial Pressure :
Making The Pressure inside the tube due to the H₂(g) the subject of the formula :
we have:
= (765 -24) torr
= 741 torr
According to ideal gas law and Dalton's law of partial pressure, 0.0136 moles of hydrogen are produced in the reaction.
What is ideal gas law?The ideal gas law is a equation which is applicable in a hypothetical state of an ideal gas.It is a combination of Boyle's law, Charle's law,Avogadro's law and Gay-Lussac's law . It is given as, PV=nRT where R= gas constant whose value is 8.314.The law has several limitations.It is applicable to ideal gases which have hypothetical existence.Law was proposed by Benoit Paul Emile Clapeyron.
In the given problem, according to Dalton's law of partial pressure, pressure =765-24=741 torr
Substituting the given values in the ideal gas equation, n=PV/RT
n=741×45.6×10[tex]^-3[/tex]/8.314×298=0.0136 moles.
Thus 0.0136 moles of hydrogen are produced in the reaction.
Learn more about ideal gas law,here:
https://brainly.com/question/28257995
#SPJ5
CAN SOMEONE PLEASE HELP ME I WILL MARK AS BRAINLIEST :(
Answers
Answer:
ok so i did the problem and the answer was 8e+29
Explanation:
i dont really know what the answer is but thats the answer i got
sorry if this didnt help
HELP WHOEVER ANSWERS WILL BE MARKED BRAINLIEST!!!
Answers
Which part of the kite catches the wind?
a Cover
b Frame
c Tail
d Kite string
Answers
Answer:
cover A
Explanation:
Answer:
cover sorry if im wrong
Explanation:
the cover makes the kiete float using air resisance
Copper has a specific heat of 0,900 J(g C). How much energy in kJ is needed to raise the temperature of a 700 g block of aluminum from 30.7°C to 82,1C?(show your steps)
Answers
Answer:
32.4 kJ
Explanation:
Given that:
Specific heat capacity fo copper, c = 0.9
Mass of copper, m = 700 g
Temperature change, dθ ;t2 - t1 = (82.1 - 30.7) = 51.4
Energy required, E = mcdθ
E = 700 * 0.9 * 51.4
E = 32382 J
Energy required on kJ
32382 / 1000
= 32.382 kJ
= 32.4 kJ
Kevin is working on a model that shows the positions of Earth, the Moon, and the Sun during the phases of
the Moon. How should he position them to show a New Moon?
Answers
Answer:
Explanation:what’s the answer
Answer: A. With Earth between the Moon and the Sun.
Explanation:
The burning of fossil fuels, like coal, oil, and natural gas, DO NOT contribute to the presence of greenhouse gases in the atmosphere.
True
or
False
Answers
Answer:
false statements because they are all nonrenewable resources
A certain protein transports a sodium ion from the inside of the cell membrane to the outside using active transport. Which of the following must be true? A. There is no change in concentration before or after transport. B. The transport process did not use chemical energy. C. After transport, the concentration of protein in the cell decreases. D. After transport, the concentration of sodium is lower inside the cell.
Answers
The energy we get from the sun is called THERMAL ENERGY and the energy from the Earth is called SOLAR RADIATION.
True
or
False
Answers
The solar radiation that reaches the Earth's surface without being diffused is called direct beam solar radiation. The sum of the diffuse and direct solar radiation is called global solar radiation. Atmospheric conditions can reduce direct beam radiation by 10% on clear, dry days and by 100% during thick, cloudy days.
Sulfur
how many electron shells in 4th shell?
Answers
Answer:
16 electrons in a sulfur atom. Looking at the picture, you can see there are two electrons in shell one, eight in shell two, and six in shell three.
Explanation:
When a 17.9 mL sample of a 0.458 M aqueous nitrous acid solution is titrated with a 0.368 M aqueous potassium hydroxide solution, what is the pH after 33.4 mL of potassium hydroxide have been added
Answers
Answer:
pH = 12.90
Explanation:
THe reaction of HNO₃ with KOH is:
HNO₂ + KOH → KNO₂ + H₂O
That means 1 mole of nitrous acid reacts per mole of potassium hydroxide.
To solve this question, we need to find the moles of each reactant:
Moles HNO₂:
0.0179L * (0.458mol / L) = 0.00820 moles
Moles KOH:
0.0334L * (0.368mol / L) = 0.01229 moles
That means KOH is in excess. The moles in excess are:
0.01229 moles - 0.00820 moles = 0.00409 moles KOH = Moles OH⁻
The [OH⁻] is -Total volume = 17.9mL+33.4mL = 51.3mL = 0.0513L-:
0.00409 moles / 0.0513L =
0.0797M =[OH⁻]
pOH = -log[OH⁻] = 1.098
pH = 14 - pOH
pH = 12.90Consider an ideal gas enclosed in a 1.00 L container at an internal pressure of 18.0 atm. If the gas expands against a constant external pressure of 1.00 atm to a final volume of 10.0 L, what is the work done?
Now calculate the work done if this process is carried out in two steps.
1. First, let the gas expand against a constant external pressure of 5.00 atm to a volume of 2.00 L.
2. From there, let the gas expand to 10.0 L against a constant external pressure of 1.00 atm.
Answers
Answer:
A=-0.9117kJ.
B= -0.5065kJ
C=-0.8104KJ
Explanation: A s gas expands, it produces works . Therefore
Work done by gas expansion,
W = P Δ V
where P =external pressure,
ΔV = change in volume (final - Initial)
W = -1 atm (10 - 1 ) L
W = -9L. atm x 101.3J/ 1L. atm
W = 911.7Joules ≈ -0.9117kJ.
1.The work done in the first step is:
W= - 5.00 atm × (2.00 L - 1.00 L) = -5 atm·L ×(101.3J / 1 atm·L)
=506.5J = -0.5065kJ
2.The work done in the second step is:
w = - 1.00 atm × (10.0 L - 2.00 L) = -8atm·L ×(101.3J / 1 atm·L
=810.4J
w=-0.8104KJ
The work done in the whole process is:
w =-0.5065kJ --0.8104KJ = -1.3169KJ
Which of these statements best supports the idea that a cell is the basic unit of a living organism?
A.
The number of cells in an organism affects the size of that organism.
B.
A tissue is composed of cells with similar structure and function.
C.
Some organisms have only one cell.
D.
All organisms are made up of one or more cells.
Answers
Answer:
The answer is D.
Explanation:
I searched it up :)
Rock is driven underground and changed by heat and pressure. This describes
what?
a. Igneous changing to sedimentary
b. Metamorphic changing to sedimentary
C. Sedimentary changing to metamorphic
d. Sedimentary changing to igneous
Answers
Answer:
Explanation:
metamorphic
How many atoms are in 1.50 moles of Zinc?
Answers
2. Nitric oxide, NO, is made from the oxidation of NH3, and the reaction is represented by the equation:
4NH3 + 502 → 4NO + 6H2O
What mass of NO can be produced from 6.82 g of NH3?
Need asap, will give brainliest
Answers
Answer:
give me branlist it is 120nh
Balance the following equation and
then provide the coefficients for each
molecule.
_SnO2+ _H2 --> _Sn + _H20
Answers
Answer:
jbisibjas bhijjoijnc
Explanation:
State the coefficient required to correctly balance the following chemical equation:
____KCl + ____Fe -----> ____FeCl2 + ____K
Answers
Answer:
2kcl+ fe= Fecl2+2K
so coefficient required be 2 for kcl and 2 forK
When 14 cal of heat are added to 12g of a liquid its temperature rises from 10.4 C to 12.7 C. What is the specific heat of the liquid
Answers
Answer:
0.51 cal/g.°C
Explanation:
Step 1: Given data
Added energy in the form of heat (Q): 14 calMass of the liquid (m): 12 gInitial temperature: 10.4 °CFinal temperature: 12.7 °CStep 2: Calculate the temperature change
ΔT = 12.7 °C - 10.4 °C = 2.3 °C
Step 3: Calculate the specific heat of the liquid (c)
We will use the following expression.
c = Q / m × ΔT
c = 14 cal / 12 g × 2.3 °C = 0.51 cal/g.°C
Cool air can hold less water vapor than warm air. Apply this fact to explain why clouds and precipitation form on the windward side of the mountain.
Answers
The pressure is directly proportional to temperature (when the pressure decrease the temperature decrease too). Because the air parcel expands so the molecules will not interact with each other as much.
The energy of the particles does not change but the fact that the particles are more spaced out means the parcel is cooler.
so now, the warmer a parcel of air the more water vapor it can hold. so, if a parcel of air cools it's ability to hold water vapor drops and if it drops to a low enough point that is when the water vapor will condense and turn back into liquid water. This is how clouds and precipitation form on the the windward side of the mountain.
Clouds and precipitation form on the windward side of the mountain due to change in temperature and pressure.
What is the relation between pressure and temperature?
Temperature of any substance is directly proportional to the pressure of that substance.
If the temperature of the wind increases so that winds get warmer and at the same time pressure of gas also increases, due to which particles will go far from each other. After this warm air rises up and cools down will condenses to form clouds, after this precipitate will falls on the windward side of the mountain.
Hence due to temperature and pressure precipitate will form on the windward side of the mountain.
To know more about temperature & pressure, visit the below link:
https://brainly.com/question/25290815
#SPJ2
Rick is creating a love potion for Morty. To make the potion, Rick's needs 51 mL of a mixture solution where 40% is carbonated water. After checking around his shop, Rick finds two solutions he could use. The first solution he found is 65% green tea, 15% carbonated water, and 20% whole milk. The second solution is 17% orange juice, 38% lemonade, and 45% carbonated water. How much of the first solution and second solution does Rick need to mix together to create the love potion? Round your final answers to one decimal place. You may solve this problem using any method we have learned in the class.
Answers
Answer:
The amount of the first solution rick needs to mix together to create the love portion is 8.5 mL
Explanation:
So as to make the love potion, we have;
The percentage of carbonated water in the love portion = 40%
The percentage of green tea in the first solution = 65%
The percentage of carbonated water in the first solution = 15%
The percentage of whole milk in the first solution = 20%
The percentage of orange juice in the second solution = 17%
The percentage of lemonade in the second solution = 38%
The percentage of carbonated water in the second solution = 45%
Let 'x' represent the volume in mL of the first solution added to make the love portion, and let 'y' be the volume in mL of the second solution added to make the love portion, we have;
x + y = 51...(1)
0.15·x + 0.45·y = 0.40×51 = 20.4
0.15·x + 0.45·y = 20.4...(2)
Solving the system of simultaneous equation by making 'y' the subject of each of the equation gives;
For equation (1)
y = 51 - x
For equation (2)
y = 20.4/0.45 - (0.15/0.45)·x = 136 - 3·x
y = 136/3 - (1/3)·x
Equating the two equations of 'y', gives;
51 - x = 136/3 - (1/3)·x
51 - 136/3 = x - (1/3)·x
17/3 = (2/3)·x
(2/3)·x = 17/3
x = (3/2) × (17/3) = 17/2 = 8.5
x = 8.5
y = 51 - x = 42.5
y = 42.5
Therefore, the amount of the first solution rick needs to mix together to create the love portion, x = 8.5 mL