Answers
Answer:
valence electrons
Explanation:
The valence electrons are the outer most electrons and the principal energy level in which they belong will vary for .
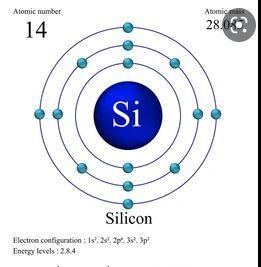
The chemical element which contains four (4) electrons in its third and outer main energy level is: Silicon (Si).
An electron shell can be defined as the outermost shell of an atom of a chemical element around the atomic nucleus.
Hence, an electron shell is an orbital that is typically accompanied by electrons around the nucleus of an atom.
A sublevel is also referred to as an orbital and it can be defined as an energy level that is associated with the electrons found outside the atomic nucleus.
In Chemistry, there are four main (4) types of sublevel and these are:
s orbital (sublevel): it has one (1) orbital i.e 1s.p orbital (sublevel): it has three (3) orbitals.d orbital (sublevel): it has five (5) orbitals.f orbital (sublevel): it has seven (7) orbitals.In the third (3rd) energy level, there are only three (3) sublevels and these are; s, p and d sublevels.
Silicon is a chemical element that is found in group (4) of the periodic table because it has four (4) electrons in its third and outermost shell.
In its ground state, Silicon (Si) contains the following number of electrons:
Two electrons in its first (n = 1) energy level. Eight electrons in the second (n = 2) energy level.Lastly, it contains four (4) electrons in its third (n = 3) and outer main energy level.Read more: https://brainly.com/question/18214726
Related Questions
A baseball strikes the roof of a car and dents it. The paint on the roof begins to crack and chip, exposing the metal. The exposed metal on the roof rusts, eventually causing a small hole in the roof. 17. Which event is a chemical change? The baseball strikes the roof The roof of the car dents The paint cracks and chips The exposed metal rusts
Answers
Answer:
The exposed metal rusts is an example of a chemical change because rust is an example of a chemical change in objects for example bicycles, scooters, etc.
which dissolved first in acetone? food coloring or liquid paint?
Answers
Answer:
Liquid paint.
Explanation:
Liquid paints are dissolved first in acetone. Most of the food dyes are not soluble in acetone.
What is acetone?Acetone is an organic compound comes under the category of ketones. It contains a carbonyl group and can dissolve most of the organic solvents.
The active ends in acetone easily forms hydrogen bonds with other solvents with polar or nonpolar groups.
Xylene, benzene, toluene, aromatic azo dyes etc. are common components in paint. Which are easily miscible with acetone.
Hence, liquid paints dissolve in acetone and food dyes are hard o dissolve in it.
To learn more about acetone, refer the link below:
https://brainly.com/question/13334667
#SPJ2
Two students apply force to a box at rest on the floor.
Left
Right
40 Newtons
30 Newtons
What is the total amount of force in Newtons acting on the box and in which direction?
10 N Left
10 N Right
• 70 N Left
70 N Right
Answers
Answer:
10 N Left
Explanation:
Given parameters;
Forces acting on the box
Left force = 40N
Right force = 30N
Unknown:
Net force on the box and the direction = ?
Solution:
Force is a pull or push on a body;
← BODY →
40N 30N
The forces acting on the body are in the opposite directions, the greater force will determine the direction of pull.
Net force = Left force - Right force = 40 - 30 = 10N
The force will be directed left with a net value of 10N
What is the percent error for the experiment if the actual density is
2.49g/mL but the experimental value is 1.47 g/mL?
Answers
Answer:
The answer is 40.96%Explanation:
The percentage error of a certain measurement can be found by using the formula
[tex]P(\%) = \frac{error}{actual \: \: number} \times 100\% \\ [/tex]
From the question
actual density = 2.49g/mL
error = 2.49 - 1.47 = 1.02
We have
[tex]p(\%) = \frac{1.02}{2.49} \times 100 \\ = 40.96385542...[/tex]
We have the final answer as
40.96 %Hope this helps you
10. The density of aluminum is 2.70 g/mL. If the mass of a piece of aluminum is 244 grams, what is the volume of the aluminum?
Answers
Answer:
The answer is 90.37 mLExplanation:
The volume of a substance when given the density and mass can be found by using the formula
[tex]volume = \frac{mass}{density} \\ [/tex]
From the question we have
[tex]volume = \frac{244}{2.7} \\ = 90.370370...[/tex]
We have the final answer as
90.37 mLHope this helps you
Calculate the following quantity: molarity of a solution prepared by diluting 49.16 mL of 0.0270 M ammonium sulfate to 525.00 mL.
Answers
Answer:
2.528x10⁻³M
Explanation:
Molarity is an unit of concentration used in chemistry. Is defined as the moles of solute per liter of solution.
To find the molarity of the solution we need to determine the moles of ammonium sulfate present in the initial 49.16mL solution and, with total volume, we can find the molarity, thus:
Moles ammonium sulfate:
49.16mL = 0.04916L * (0.0270 moles / L) =
1.327x10⁻³moles ammonium sulfate
These moles are present in 525.0mL = 0.525L. Thus, molarity of the solution will be:
1.327x10⁻³moles ammonium sulfate / 0.525L =
2.528x10⁻³MTrue or False: All cells have different basic chemical composition. *
True
False
Answers
Which statement gives an advantage of multicellular organisms?
A. Cells are able to provide support
B. They can reproduce quickly
C. Their size allows them to maintain structure easily
D. They need small amounts of energy
Answers
Answer:
A. Cells are able to provide support
The term organic does not mean “natural” true or false
Answers
Organic foods are grown without artificial pesticides .
HELPP GIVING AWAY 30 POINTS!!!!!
Determine Your family wants to use
renewable energy to heat your home. Which
renewable energy resource is best suited to
your area? Explain your answer.
Answers
Answer:
wood
Explanation:
i can take the seds from the trees to row new trees and still use the wood without causing deforestation
How many 20 mL extractions of organic solvent are needed to extract at least 99.5% of 50.0 g Compound A from 100 mL of water if K
Answers
Answer:
5 extractions to extract at least 99.5% of 50.0 g Compound A from 100 mL of water
Explanation:
If K=10...
Partition coefficient is defined as the ratio of concentrations of a compound A in two inmiscibles solvents:
K = 10 = Conc. Organic solvent / Conc. Water
Usually organic phase over aqueous phase.
In the first 20mL extraction, the organic solvent will extract:
10 = X / 20mL / (50.0g - X) / 100mL
10 = 100X / (1000-20X)
10000 - 200X = 100X
10000 = 300X
X = 33.3g of compound A are extracted in the first extraction
Remember you want to extract 99.5%, that is 50.0g*99.5% = 49.75g
In the aqueous phase remain: 50-33.3g = 16.7g:
Second extraction:
10 = X / 20mL / (16.7g - X) / 100mL
10 = 100X / (334-20X)
3340 - 200X = 100X
3340 = 300X
11.1g are extracted and will remain: 16.7g - 11.1g = 5.6g
Third extraction:
10 = X / 20mL / (5.6g - X) / 100mL
10 = 100X / (112-20X)
1120 - 200X = 100X
1120 = 300X
3.8g are extracted and will remain: 5.6g - 3.8g = 1.8g
Fourth extraction:
10 = X / 20mL / (1.8g - X) / 100mL
10 = 100X / (36-20X)
360 - 200X = 100X
360 = 300X
1.2g are extracted and will remain: 1.8g -1.2g = 0.6g
Fifth extraction:
10 = X / 20mL / (0.6g - X) / 100mL
10 = 100X / (12-20X)
120 - 200X = 100X
120 = 300X
0.4g are extracted. The total extractions gives:
33.3g + 11.1g + 3.8g + 1.2g + 0.4g = 49.8g
That means, you need to do:
5 extractions to extract at least 99.5% of 50.0 g Compound A from 100 mL of waterHELPPPPP
Identify whether each of the following changes is a physical change or a chemical change. Write “P” on the line for a physical change and a “C” for a chemical change.
22. Water boiling ______23. Iron rusting ______24. Butter melting _____25. Wood rotting ______
26.Alcohol evaporating _____27.Glass breaking ______28.Mowing the lawn ______29.Baking a cake ______
Answers
Answer:
22 is P
23 is C
24 is P
25 is C
26 is P
27 is P
28 is P
29 is C because youre using thermal heat and you cannot return to raw batter
which one of these best defines climate
please help i will mark brainlest answer if correct asap
Answers
Answer:
Long term condition of the atmosphere
Explanation:
I think this is right.
I hope this helps! (✿◕‿◕✿)
A student was performing a separation of a mixture of organic compounds. The final step of the process involved a filtration of the analyte from an aqueous solution. After drying the filtered solid for a very short period time, they took the melting point of the compound. The measured melting point range of the compound was 106 – 113.8 0C, while the literature melting point of the compound was 122.3 0C. The above scenario is a very common one in organic labs.
1. Do you think their sample was pure?
2. If not, then what do you think could be the source of error.
3. How do you think this error can be minimized?
Answers
Answer:
1) No
2) The solvent contaminated the analyte
3) The solvent should be evaporated properly before washing and drying the analyte
Explanation:
During separation of organic compounds, solvents are used. These solvents are able to contaminate the analyte and lead to a large difference in melting point of solids obtained.
However, the error can be minimized by evaporating the solvent before washing, drying and melting point determination of the solid.
A species that has 13 protons and 10 electrons will be_____
Answers
Answer: Aluminum
Explanation:
Al3+ indicates an ion of aluminum having a charge of + 3. I.e., since an aluminum atom normally has 13 protons and 13 electrons, this ion has 10 electrons (-10 charge) and 13 protons (+ 13 charge) giving it a charge of + 3 (-10 + 13 = +3).
Answer:
Explanation:
Al3+ indicates an ion of aluminum having a charge of + 3. I.e., since an aluminum atom normally has 13 protons and 13 electrons, this ion has 10 electrons (-10 charge) and 13 protons (+ 13 charge) giving it a charge of + 3 (-10 + 13 = +3).
ccto.
What is the final concentration if water is added to 0.25 L of a 8M NaOH solution to make 4.0 L of a diluted NaOH solution?
.25 M NaOH solution
1 M NaOH solution
.5 M NaOH solution
Answers
Answer:
0.5 M NaOH solution
Explanation:
Initially, we have 0.25L or 250 mL of 8M NaOH solution
Which means that 1L of this solution would have 8 moles of NaOH
Moles present in 250 mL of solution:
Molarity * Volume( in L)
8 * 0.25 = 2 moles
There is 2 moles of NaOH in 0.25 L of the provided NaOH solution
More water is going to be added to this solution but the number of moles of NaOH will remain the same
now that the solution has a volume of 4L after adding the additional water, we have a 4L solution which contains 2 moles of NaOH
Molarity of the new solution:
Molarity = Number of moles in solution / Volume of solution
Molarity = 2 / 4
Molarity = 0.5 M
A sample of an unknown gas weighs 0.419 grams and produced 5.00L of gas at 1.00atm (unknown gas only) and 298.15K, what is the molar mass (g/mole) of this unknown gas
Answers
Answer:
molar mass of unknown gas = 1.987 g/mol
Explanation:
First, the number of moles of the unknown gas is found
Using the ideal gas equation: PV = nRT
P = 1.00 atm, V = 5.00 L, T = 298.15 K, R = 0.082 L.atm.mol⁻¹K⁻¹
n = PV/RT
n = (1.00 atm * 5.00 L)/(298.15 K *0.082 L.atm.mol⁻¹K⁻¹)
n = 0.2109 moles
Molar mass = mass/ number of moles
molar mass = 0.419 g/ 0.2109 mols
molar mass of unknown gas = 1.987 g/mol
The molar mass of unknown gas by using ideal gas equation = 1.987 g/mol.
Ideal gas equationThis equation gives the relation between pressure, volume, temperature as given below:
[tex]PV = nRT[/tex]
P = 1.00 atm, V = 5.00 L, T = 298.15 K, R = 0.082 L.atm.mol⁻¹K⁻¹
Substitute the above values in the above equation as follows:
n = (1.00 atm * 5.00 L)/(298.15 K *0.082 L.atm.mol⁻¹K⁻¹)
n = 0.2109 moles
Formula for molar mass[tex]Molar mass = mass/ number of moles[/tex]
Calculate molar mass by using the above equation,
molar mass = 0.419 g/ 0.2109 moles
The molar mass of unknown gas = 1.987 g/mol
Find more information about ideal gas equation here,
brainly.com/question/4147359
Which neutral atom is isoelectronic with Cl-??
Answers
And we can see that the potassium ion, K+, has the same electronic configuration as the chloride ion, Cl-, and the same electronic configuration as an atom of argon, Ar. Therefore, Ar, Cl-, and K+ are said to be isoelectronic species.
CHEMISTRY!! 50 POINTS!
There are 5.5 L of a gas present at -38.0 C. What is the temperature if the volume of the gas has changed to 1.30 L?
Answers
Answer:
The answer to this question is 33.8
Based on the visible cell structures, which of the following statements is true?
All of the cells are plant cells.
All of the cells have chloroplasts.
All of the cells are animal cells.
All of the cells have a nucleus.
Answers
According to Avogadro's law, what is characteristic of 1 mole of gas at STP?
A. It occupies 22.4 L.
B. It occupies no volume.
C. It occupies 1 L.
D. It will expand to any volume.
Answers
Answer:
A. It occupies 22.4 L
Explanation:
STP (Standard Conditions for Temperature and Pressure) = 22.4 L per mole at 1 atm
Avogadro's Law states that 1 mol at 1 atm occupies 22.4 L.
Be sure to answer all parts.
Write a balanced equation for each reaction. Do not include states of matter to your equation.
H2 + O2 H20
Answers
Answer:
2H₂ + O₂ → 2H₂O
Explanation:
Chemical equation:
H₂ + O₂ → H₂O
Balance chemical equation:
2H₂ + O₂ → 2H₂O
Step 1:
H₂ + O₂ → H₂O
Left hand side Right hand side
H = 2 H = 2
O = 2 O = 1
Step 2:
H₂ + O₂ → 2H₂O
Left hand side Right hand side
H = 2 H = 4
O = 2 O = 2
Step 3:
2H₂ + O₂ → 2H₂O
Left hand side Right hand side
H = 4 H = 4
O = 2 O = 2
The smallest unit of an element that can exist either alone or in combination with other such particles of the same or different elements is the
А.
electron
B
proton
neutron
D
atom
Answers
Answer:
The answer is D - Atom
The smallest unit of an element that can exist either alone or in combination with other such particles of the same or different elements is the atom.
What is an atom?
An atom is defined as the smallest unit of matter which forms an element. Every form of matter whether solid,liquid , gas consists of atoms . Each atom has a nucleus which is composed of protons and neutrons and shells in which the electrons revolve.
The protons are positively charged and neutrons are neutral and hence the nucleus is positively charged. The electrons which revolve around the nucleus are negatively charged and hence the atom as a whole is neutral and stable due to presence of oppositely charged particles.
Atoms of the same element are similar as they have number of sub- atomic particles which on combination do not alter the chemical properties of the substances.
Learn more about atom,here:
https://brainly.com/question/13654549
#SPJ2
why are copper pipes used in place of old lead pipes for plumbing systems?
Answers
20
How do you determine the number of barium atoms in the formula below?
4Ba(OH)2
F
# of Ba atoms = coefficient 4 + subscript 1 = 5
G
# of Ba atoms = coefficient 4 X subscript 1 = 4
H
# of Ba atoms = subscript 4 X coefficient 2 = 6
# of Ba atoms coefficient 1 + subscript 1 = 2
J
Answers
Answer:
G is correct option:
# of Ba atoms = coefficient 4 × subscript 1= 4
Explanation:
Formula:
4Ba(OH)₂
G is correct option:
# of Ba atoms = coefficient 4 × subscript 1= 4
Because there are only 4 atoms of Ba in given formula.
Ba(OH)₂ contain one atom of Ba while in question there are 4 moles of Ba(OH)₂ present thus total 4×1 = 4 atoms of Ba present.
Other options are incorrect. Because,
F:
# of Ba atoms = coefficient 4 + subscript 1 = 5
This shows given formula contain 5 Ba atoms. So it is incorrect.
H:
# of Ba atoms = subscript 4 × coefficient 2 = 6
This shows that subscript is 4 which is incorrect because coefficient is 4 and subscript is 1.
j:
# of Ba atoms = subscript 1 + coefficient 1 = 2
This option shows that subscript is one which is correct but coefficient is incorrect. The coefficient of Ba is 4 and coefficient is always multiply with subscript not added. So this option is also incorrect.
The incredible catalytic power of enzymes can perhaps best be appreciated by imagining how challenging life would be without just one of the thousands of enzymes in the human body. For example, consider life without fructose-1,6-bisphosphatase, an enzyme in the gluconeogenesis pathway in liver and kidneys, which helps produce new glucose from the food we eat:
Fructose-1,6-bisphosphate + H2O → Fructose-6-phosphate + Pi
The human brain requires glucose as its only energy source, and the typical brain consumes about 120. g (or 480. calories) of glucose daily. Ordinarily, two pieces of sausage pizza could provide more than enough potential glucose to feed the brain for a day. According to a national fast-food chain, two pieces of sausage pizza provide 1260 calories, 49.0 % of which is from fat. Fats cannot be converted to glucose in gluconeogenesis, so that leaves 615 calories potentially available for glucose synthesis. The first-order rate constant for the hydrolysis of fructose-1,6-bisphosphate in the absence of enzyme is 2.00×10-20 sec-1.Calculate how long it would take to provide enough glucose for one day of brain activity from two pleces of sausage pizza without the enzyme.
Answers
Answer:
t = 7.58 * 10¹⁹ seconds
Explanation:
First order rate constant is given as,
k = (2.303 /t) log [A₀] /[Aₙ]
where [A₀] is the initial concentraion of the reactant; [Aₙ] is the concentration of the reactant at time, t
[A₀] = 615 calories;
[Aₙ] = 615 - 480 = 135 calories
k = 2.00 * 10⁻²⁰ sec⁻¹
substituting the values in the equation of the rate constant;
2.00 * 10⁻²⁰ sec⁻¹ = (2.303/t) log (615/135)
(2.00 * 10⁻²⁰ sec⁻¹) / log (615/135) = (2.303/t)
t = 2.303 / 3.037 * 10⁻²⁰
t = 7.58 * 10¹⁹ seconds
Calculate the sublimation pressure of the solid at the melting point of 400.00 K assuming that the enthalpy of sublimation is not a function of temperature.
Answers
This question is incomplete, the complete question is;
Tonksite is a solid at 300.00K. At 300.00 K its enthalpy of sublimation is 66.00 kJ/mol. The sublimation pressure at 300.00 K is 5.00 × 10⁻⁴ atm
Calculate the sublimation pressure of the solid at the melting point of 400.00 K assuming that the enthalpy of sublimation is not a function of temperature.
Answer: the sublimation pressure of the solid at the melting point is 0.3727 atm
Explanation:
Given that;
T1 = 300 K
T2 = 400 K
H_sub = 66 kJ/mol = 66000 J/mol
P1 = 5.00 × 10⁻⁴ atm
p2 = ?
now using the expression
log( p2 / 5.00 × 10⁻⁴ ) = (H_sub / R × 2.303 ) (( T2 - T1) / T1T2)
now we substitute of given values into the expression
log(p2/p1) = (66000 / 8.314 × 2.303 ) (( 400 - 300) / 300 × 400 )
p2 = 0.3727 atm
therefore the sublimation pressure of the solid at the melting point is 0.3727 atm
A factory discharges clean, warm water into a nearby stream. Fish keep dying in this part of stream. Explain why.
Answers
Answer:
the fish can't survive in that warm water
Explanation:
when the factory puts that warm water into the stream where the fish live they changed their environment. by adding unknown chemicals and changing the temp of the water the fish start to die.
The fish aren't used to living in that warm water, and if they can't adapt fast enough they will die, also the unknown chemicals that could be in the water will act as a poison for them making that stream unable to support any life.
8. Besides landforms, the other large features that cover the
surface of the earth are ______.
A. islands
B. waterways
C. deserts
D. lakes
Answers
Answer:
A landform is a feature on the Earth's surface that is part of the terrain. Mountains, hills, plateaus, and plains are the four major types of landforms. Minor landforms include buttes, canyons, valleys, and basins. Tectonic plate movement under the Earth can create landforms by pushing up mountains and hills.
Explanation:
"Waterways" are the other large features that cover the surface of the earth.
Waterways
A canal would be any body of water throughout which boats may move. Waterways, on the other hand, comprise waterways that seem to be expansive as well as shallow enough already to allow freight-carrying watercraft just to travel through.
Some bodies of water are usually regularly dredged to maintain an appropriately deep course throughout all circumstances. Dams change the depth of other rivers in particular parts.
Thus the response above i.e., "Option B" is correct.
Find out more information about landform here:
https://brainly.com/question/1920109
I don't know what category to put this question in, but I attached a photo of it. Can someone please help me answer it?
Answers
Analysing the question:
To calculate the density of a material, we need its mass and volume
We are given:
Mass of sample = 21 grams
dimensions of the sample = 1 * 1 * 2 = 2 cm³
Calculating the density:
Density = Mass of sample / volume of sample
Replacing the variables
Density = 21 / 2
Density = 10.5 g / cm³
Determining the Material:
From the table provided, we can see that the density of Silver is 10.5 g/cm³
Therefore, the material is Silver