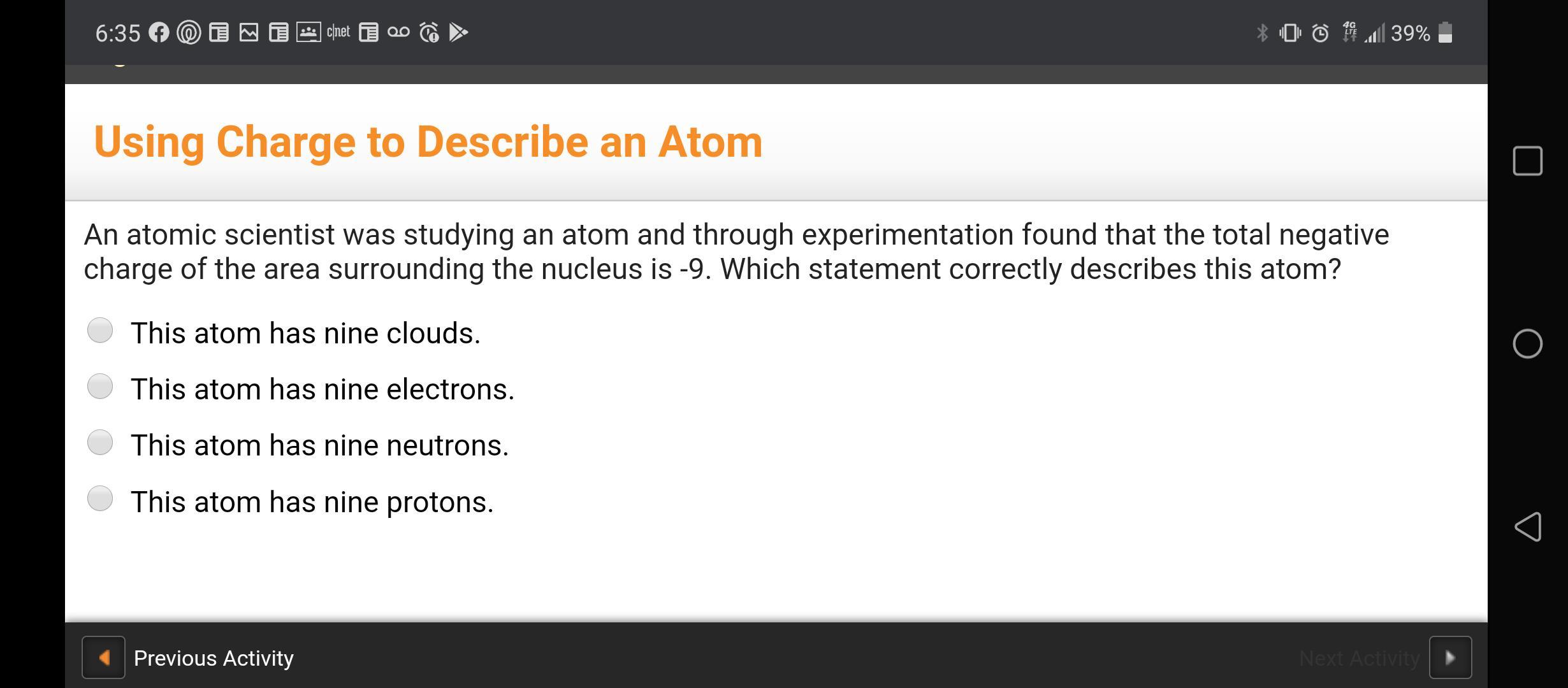
Answers
Answer:
this atom has nine electrons
Related Questions
What metalloid has commonly been used as an insecticide due
to its effectiveness as a poison.
Answers
Answer:
Arsenic.
Explanation:
Hello there!
In this case, since insecticides are substances that act as poisons to get rid of insects in order to prevent their presence and/or reproduction in houses, companies, crops and others, a substance that has been widely used is the metalloid arsenic due to its direct affection of the insect's body (movement, performance, cellular functions).
In addition, high levels of arsenic in food could cause arsenic poisoning in humans as well, that is why such practice must be properly performed and by using the correct security protocol.
Best regards!
Answer:
As
Explanation:
hoffe das hilf jedem in der zukunft
the egents erosion and explain how each of them causes erosion
Answers
Answer:
Wind, water, gravity, and ice
Explanation:
Water can erode soil material. Especially if the soil is bare, dry and erodible erosion via rain particles can occur. Wind is another factor that cause soil erosion. Dry soil particles( Especially if they are fine) can move to other areas if wind exists. Ice is another issue on erosion. Ice, when it is melting, can carry soil particles.
Which of the following is true?
A) The closer an electron is to the nucleus, the higher its energy level.
B) The closer an electron is to the nucleus, the lower its energy level.
C) The closer an electron is to the nucleus, the more its energy level fluctuates.
D) There is no relationship between proximity to the nucleus and the energy level of an electron
Answers
Answer:
B) The closer an electron is to the nucleus the lower it's energy level.
Explanation:
This is because the attractive force for electrons to the nucleus is stronger. Thus, the orbital energy becomes less.
Level 1 01 Which correctly pairs the outside particles with their charge? A. Electrons: Positive B. Protons: Positive C. Neutrons: Neutral D. Electrons: Negative
Answers
Answer:
D. Electrons: Negative.
Explanation:
Hello, happy to help you today!
In this case, by considering the Bohr's atomic model in which atom is composed by a nuclei containing both protons and neutrons which are positively and neutrally charged respectively and surrounding electrons assembled in orbits or levels of energy which are negatively charged in order to provide a balance to the atom, the correct statement is: D. Electrons: Negative. Also consider the Bohr's model on the attached picture.
My best regards to you!
10. Predict the mass of nitrogen dioxide produced if 2.30 L of ammonia are allowed to react
with excess oxygen gas at STP?
Answers
Answer:
Mass of nitrogen dioxide produced = 4.6 g
Explanation:
Given data:
Volume of ammonia = 2.30 L
Mass of nitrogen dioxide produced = ?
Solution:
Chemical equation:
4NH₃ + 7O₂ → 4NO₂ + 6H₂O
Number of moles of ammonia at STP:
PV = nRT
n = PV/RT
n = 1 atm × 2.30 L / 0.0821 atm.L/K.mol × 273 K
n = 2.30 atm .L / 22.414 atm.L/mol
n = 0.1 mol
Now we will compare the moles of ammonia with nitrogen dioxide from balance chemical equation.
NH₃ : NO₂
4 : 4
0.1 : 0.1
Mass of NO₂:
Mass = number of moles × molar mass
Mass = 0.1 mol × 46 g/mol
Mass = 4.6 g
Rank these systems in order of decreasing entropy. Rank from highest to lowest entropy.
a. 1 mol carbon tetrafluoride gas at 273k 40L
b. 1 mol krypton gas at 273K 40L
c. 1/2 mol krypton gas at 100k 20L
d. 1 mol krypton gas at 273K 20L
e. 1/2 mol krypton liquid at 100K
f. 1 mol fluorine gas 273 K 40L
g. 1/2 mol krypton gas at 273K 20L
Answers
Answer:
The answer is "order will be A > F > B> D > G > C > E".
Explanation:
Entropy is disunity, the greater the lack of organization the entropy. When two gases use the same moles, then more entropy is achieved for a larger number of atoms in the molecule (even with more macrostates). Its smaller your volume the less the molecules may circulate, reducing as well as the amount of potential different countries and thus the entropy. Molecules or atoms in colder gas are much less active so they do not actually take so many various energy states and therefore less entropy. In colder gas. A liquid is requested more so than gas and its randomness decreases.Is a diamond a homogeneous or heterogeneous mixture or a substance
Answers
Answer:
heterogeneous
Explanation:
It's a heterogeneous mixture. Diamond is made of just one element: carbon. Each carbon atom in diamond is connected to four other carbon atoms, in a crystal that extends on and on. There are other forms of pure carbon where the atoms are bonded differently, notably charcoal and graphite.
Plz help me with this if you give me a good answer i will give you eaxtra points plz help its do today
Answers
Answer:
Generally described, the input/output device includes a housing for one or more input components such as a keyboard and/or microphone, one or more output components such as a display screen or a speaker, a data storage device such as a hard drive, a processor, and a phone interface.
An input mechanism to allow the user to interact with the phone. The most common input mechanism is a keypad, but touch screens are also found in smartphones. Basic mobile phone services to allow users to make calls and send text messages. All GSM phones use a SIM card to allow an account to be swapped among devices.
The maximum possible power output of a handheld cellular phone is 0.6 watts. Like others have posted, the system controls the actual output of the phone and it is typically much less than that, usually less than 100 milliwatts.
Explanation:
If an object has a density of 6.05 g/cm3 and a volume of 36.5 cm3, what is its mass?
Answers
Answer:
0.42 g/cm3
Explanation:
Answer:
20/40=0.5 g/cm^3 becuase, mass/volume=density
Explanation:
hello I am working on naming compounds and wondering if you could help me figure out the name
Answers
Al2(SO4)3 aluminium sulfate
Calcium reacts with sulfur forming calcium sulfide. What is the theoretical yield (g) of CaS(s) that could be prepared from 7.19 g of Ca(s) and 2.67 g of sulfur(s)? Enter your answer with two decimal places. Do not type units with your answer.
Answers
Answer:
The theoretical yield of CaS is 6.01 g.
Explanation:
The balanced reaction is given as:
[tex]Ca+S\rightarrow CaS[/tex]
The molar mass of Ca and S is 40.08 and 32.065 g/mol respectively.
Number of moles = [tex]\frac{Mass}{Molar Mass}[/tex]
So, 7.19 g of Ca contains [tex](\frac{7.19}{40.08})[/tex] mol of Ca or 0.179 mol of Ca
Also, 2.67 g of S contains [tex](\frac{2.67}{32.065})[/tex] mol of S or 0.0833 mol of S
According to the balanced equation:
1 mol of Ca produces 1 mol of CaS
So, 0.179 mol of Ca produces 0.179 mol of CaS
According to the balanced equation:
1 mol of S produces 1 mol of CaS
So, 0.0833 mol of S produces 0.0833 mol of CaS
As the least number of mol of CaS (product) is produced from S , therefore, S is the limiting reactant.
So, thoretically, 0.0833 mol of CaS is produced.
The molar mass of CaS is 72.143 g/mol.
So, the mass of 0.0833 mol of CaS is [tex](0.0833\times 72.143)[/tex] g or 6.01 g
Hence, the theoretical yield of CaS is 6.01 g.
Select from the drop-down menu to correctly complete the statement. Cells that perform a specific function in an organism Choose... .
A. Can survive on their own
B. Are specialized
C. Perform all of the. function
D. are all the same
Answers
Answer:
B :)
Explanation:
The cells that perform the specific function in an organism so it should be specialized.
What are specialized cells?
It is the cell that should be made up of tissued also with the system so that it should be working together in our bodies. It includes the examples like blood cells, nerve cells, reproductive cells. Also, they have their own functions.
Therefore, we can conclude that The cells that perform the specific function in an organism so it should be specialized.
Hence, the option b is correct.
Learn more about cell here: https://brainly.com/question/24022029
Assuming constant pressure, rank these reactions from most energy released by the system to most energy absorbed by the system, based on the following descriptions:
Surroundings get colder and the system decreases in volume.
Surroundings get hotter and the system expands in volume.
Surroundings get hotter and the system decreases in volume.
Surroundings get hotter and the system does not change in volume.
A mole of X reacts at a constant pressure of 43.0 atm via the reaction.
X(g)+4Y(g)→2Z(g), ΔH∘=−75.0 kJ
Also assume that the magnitude of the volume and temperature changes are similar among the reactions. Rank from most energy released to most energy absorbed. To rank items as equivalent, overlap them. View Available Hint(s)
Answers
Answer:
The options are
A.Surroundings get colder and the system decreases in volume.
B.Surroundings get hotter and the system expands in volume.
C.Surroundings get hotter and the system decreases in volume.
D.Surroundings get hotter and the system does not change in volume.
From the Most energy released to the most absorbed , the order is
B. Surroundings get hotter and the system expands in volume.
D. Surroundings get hotter and the system does not change in volume.
C. Surroundings get hotter and the system decreases in volume.
A. Surroundings get colder and the system decreases in volume.
2NH.
N2 + 3H2
Reactants
Product
On the balanced equation above, how many
atoms of each element are in the reactant?
N =
H =
Answers
Answer:
N=2
H=6
Explanation:
1.Balance a chemical equation in terms of moles.
2.Use the balanced equation to construct conversion factors in terms of moles.
3.Calculate moles of one substance from moles of another substance using a balanced chemical equation.
The law of conservation of matter says that matter cannot be created or destroyed. In chemical equations, the number of atoms of each element in the reactants must be the same as the number of atoms of each element in the products.
(P.s it could also be where you have to solve it in which you have to simplify it first then solve it.) like adding them all up.
Hope this is the answer. :)
A solution of 0.050 M benzoic acid, HC7H5O2 , is 3.5% ionized. at 25 oC. a) What are the [H ] and pH of this solution
Answers
Answer:
[H⁺] = 0.00175 M
pH = 2.757
Explanation:
The ionization of benzoic acid is given below:
C₆H₅COOH ----> C₆H₅COO⁻(aq) + H⁺(aq)
1 mole of H⁺ ions are produced from the dissociation of 1 mole C₆H₅COOH.
Therefore 0.050 M benzoic acid produces 0.050 M H⁺.
However, the benzoic acid is only 3.5% ionized, therefore concentration of H+ ion, [H⁺] = 3.5/100 * 0.050 M
[H⁺] = 0.00175 M
pH = - log[H⁺]
pH = - log(0.00175)
pH = 2.757
Calculate the mass in grams for 3.28 moles of O2? Please show your work to receive credit.
Answers
Answer:
105 g O₂
Explanation:
Step 1: Define
Molar Mass O - 16.00 g/mol
Molar Mass O₂ - 2(16.00) = 32.00 g/mol
Step 2: Use Dimensional Analysis
[tex]3.28 \hspace{3} mol \hspace{3} O_2(\frac{32 \hspace{3} g \hspace{3} O_2}{1 \hspace{3} mol \hspace{3} O_2} )[/tex] = 104.96 g O₂
Step 3: Simplify
We are given 3 sig figs.
104.96 g O₂ ≈ 105 g O₂
True or false: BrF5(aq) is a good insulator.
Answers
Answer:
true
hope this helps :)
Explanation:
True, [tex]BrF_5[/tex] (aq) is a good insulator.
What is an insulator?A material or an object that does not easily allow heat, electricity, light, or sound to pass through it.
Bromine pentafluoride appears as a colourless, fuming liquid with a pungent odour. Used to make other chemicals and in rockets.
Hence, [tex]BrF_5[/tex] (aq) is a good insulator.
Learn more about insulators here:
https://brainly.com/question/24909989
#SPJ2
50 POINTS!
There are 5.5 L of a gas present at -38.0 C. What is the temperature if the volume of the gas has changed to 1.30 L?
Answers
unit volume/temperature x searching amount
i’d say the temperature would be -8.98 C simply - I don’t know what formula youd use for this
Identify the correct net ionic equation for the reaction that occurs when solutions of Pb(NO3)2 and NH4Cl are mixed.
Answers
Answer:
Pb2+(aq) + 2Cl–(aq) ----> PbCl2(s)
Explanation:
The net ionic equation shows the main reaction that takes place in a system. Hence, a net ionic equation focusses only on those species that actually participate in the reaction.
For the reaction between Pb(NO3)2 and NH4Cl , the net ionic equation is;
Pb^+(aq) + 2Cl^-(aq) ---> PbCl2(s)
The correct net ionic equation for the reaction that occurs when solutions of Pb(NO₃)₂ and NH₄Cl are mixed is
Pb⁺(aq) + 2Cl¯(aq) —> PbCl₂(s)To know the the correct net ionic equation for the reaction that occurs when solutions of Pb(NO₃)₂ and NH₄Cl are mixed, we shall write the net ionic equation for the reaction. This is illustrated below:
Pb(NO₃)₂(aq) —> Pb⁺(aq) + NO₃¯(aq)
NH₄Cl(aq) —> NH₄⁺(aq) + Cl¯(aq)
Pb(NO₃)₂(aq) + NH₄Cl(aq) —>
Pb⁺(aq) + NO₃¯(aq) + NH₄⁺(aq) + Cl¯(aq) —> PbCl₂(s) + NO₃¯(aq) + NH₄⁺(aq)
Cancel the spectator ions (i.e NO₃¯ and NH₄⁺) and write 2 before Cl¯ to obtain the net ionic equation as shown below:
Pb⁺(aq) + 2Cl¯(aq) —> PbCl₂(s)Thus, the correct net ionic equation for the reaction that occurs when solutions of Pb(NO₃)₂ and NH₄Cl are mixed is
Pb⁺(aq) + 2Cl¯(aq) —> PbCl₂(s)Learn more: https://brainly.com/question/21883718
Help
Save & Exit
Submit
Rank the following elements in order from least to most number of moles of atoms in a 10.0 g sample: Sn, Si, Se, S
Answers
Answer:
[tex]\rm Sn[/tex], [tex]\rm Se[/tex], [tex]\rm S[/tex], [tex]\rm Si[/tex].
Explanation:
The relative atomic mass of an element is numerically equal to the mass (in grams) of one mole of its atoms. This quantity can help estimate the number of moles of atoms in each of these four [tex]10.0\; \rm g[/tex] samples.
Look up the relative atomic mass for each of these four elements (on a modern periodic table.)
[tex]\rm Si[/tex]: [tex]28.085[/tex].[tex]\rm S[/tex]: [tex]32.06[/tex].[tex]\rm Se[/tex]: [tex]78.971[/tex].[tex]\rm Sn[/tex]: [tex]118.710[/tex].The relative atomic mass of [tex]\rm Si[/tex] is (approximately) [tex]28.085[/tex]. Therefore, the each mole of silicon atoms would have a mass of approximately [tex]28.085\; \rm g[/tex]. How many moles of silicon atoms would there be in a [tex]10.0\; \rm g[/tex] sample?
Given:
[tex]m(\rm Si) = 10.0\; \rm g[/tex]. [tex]M(\mathrm{Si}) = 28.085\; \rm g \cdot mol^{-1}[/tex].Number of mole of silicon atoms in the sample: [tex]\displaystyle n(\mathrm{Si}) = \frac{m(\mathrm{Si})}{M(\mathrm{Si})} = \frac{10.0\; \rm g}{28.085\; \rm g \cdot mol^{-1}}\approx 0.356\; \rm mol[/tex].
Similarly:
[tex]\displaystyle n(\mathrm{S}) = \frac{m(\mathrm{S})}{M(\mathrm{S})} = \frac{10.0\; \rm g}{32.06\; \rm g \cdot mol^{-1}}\approx 0.312\; \rm mol[/tex].
[tex]\displaystyle n(\mathrm{Se}) = \frac{m(\mathrm{Se})}{M(\mathrm{Se})} = \frac{10.0\; \rm g}{78.971\; \rm g \cdot mol^{-1}}\approx 0.127\; \rm mol[/tex].
[tex]\displaystyle n(\mathrm{Sn}) = \frac{m(\mathrm{Sn})}{M(\mathrm{Sn})} = \frac{10.0\; \rm g}{118.710\; \rm g \cdot mol^{-1}}\approx 0.0842\; \rm mol[/tex].
Therefore, among these [tex]10.0\; \rm g[/tex] samples:
[tex]n(\mathrm{Sn}) < n(\mathrm{Se}) < n(\mathrm{S}) < n(\mathrm{Si})[/tex].
It is not a coincidence that among these four samples, the one with the fewest number of atoms corresponds to the element with the largest relative atomic mass.
Consider two elements, with molar mass [tex]M_1[/tex] and [tex]M_2[/tex] each. Assume that [tex]n_1[/tex] moles and [tex]n_2[/tex] moles of atoms of each element were selected, such that the mass of both samples is [tex]m[/tex]. That is:
[tex]m = n_1\cdot M_1[/tex].
[tex]m = n_2\cdot M_2[/tex].
Equate the right-hand side of these two equations:
[tex]n_1 \cdot M_1 = n_2\cdot M_2[/tex].
[tex]\displaystyle \frac{n_1}{n_2} = \frac{M_2}{M_1} = \frac{1/M_1}{1/M_2}[/tex].
In other words, the number of moles atoms in two equal-mass samples of two elements is inversely proportional to the molar mass of the two elements (and hence inversely proportional to the formula mass of the two elements.) That explains why in this question, the sample containing the smallest number of atoms corresponds to element with the largest relative atomic mass among those four elements.
What is the percent of iron and oxygen in iron(III) oxide?
Answers
Answer:
69.94% iron by mass.
Explanation:
Cations are always
Question Blank
than the neutral atom.
Answers
1. Smaller than the neutral atom due to lost electrons
2. Cations are always more positively charged than the neutral atom due to a loss of electrons.
Please mark me as brainliest answer!
If 25.6 mL isopropyl alcohol fully decomposes, what mass of H2 is formed? The density of isopropyl alcohol is 0.785 g/mL. g
Answers
Answer:
The correct answer is 0.67 g H₂
Explanation:
Isopropyl alcohol (C₃H₇OH) can decompose to give acetone (C₂H₆OH) and hydrogen gas (H₂) according to the following chemical equation:
C₃H₇OH (g) ⇒ C₂H₆CO(g) + H₂(g)
We can calculate the initial mass of isopropyl alcohol from the density and volume data:
density = m/V = 0.785 g/mL
⇒ m = density x V = 0.785 g/mL x 25.6 mL = 20.096 g C₃H₇OH
According to the chemical equation 1 mol of C₃H₇OH gives 1 mol H₂. The molar mass of C₃H₇OH is:
molar mass C₃H₇OH = (12 g/mol x 3) + (1 g/mol x 7) + 16 g/mol + 1 g/mol = 60 g/mol
molar mass H₂ = 1 g/mol x 2 = 2 g/mol
So, we obtain: 2 g H₂ from 60 g C₃H₇OH. We multiply this stoichiometric ratio (2 g H₂/60 g C₃H₇OH) by the initial mass of C₃H₇OH to obtain the mass of H₂ is formed:
20.096 g C₃H₇OH x (2 g H₂/60 g C₃H₇OH) = 0.6698 g ≅ 0.67 g H₂
17. What is the average atomic mass of the following isotopic mixture - 22.00% of 159.3 g/mole; 78.00% of
161.2g/mole?
Answers
34.046 + 125.736 = 159.8 g/mole
The average atomic mass is given by the individual atomic masses of the isotope of the element and its percentage. The average atomic mass of the isotopic mixture is 159.8 g/mole.
What are isotopes?Isotopes are atoms of the same element that have the same number of protons in their nucleus but have a different number of neutrons that alters their atomic masses. The relative abundance of the isotope of the element affects the average atomic mass of the mixture.
The formula for average atomic mass for the mixture of isotopes is given as:
Average atomic mass = ∑ (mass × abundance)
Given,
Abundance of isotope 1 = 22.00 %
Mass of isotope 1 = 159.3 g/mole
Abundance of isotope 2 = 78.00 %
Mass of isotope 2 = 161.2g/mole
Substituting values in the formula of average atomic mass as:
Average atomic mass = isotope 1 (mass × abundance) + isotope 2 (mass × abundance)
= (0.22) × (159.3) + (0.78) × (161.2)
= 34.046 + 125.736
= 159.8 g/mole
Therefore, the average atomic mass of the mixture of the two isotopes is 159.8 g/mole.
Learn more about isotopes and average mass, here:
https://brainly.com/question/2159355
#SPJ2
Products made of plastic can last a very long time. Explain both the positive and negative effects of plastic being long-lasting. thank uuu
Answers
Answer:Because plastic products are durable, they can last for a long time. This makes them affordable because they do not have to be replaced often. On the other hand, the popular use of plastics means that many plastics are thrown away. Plastics litter the ocean, causing harm to marine birds and mammals. Plastic breaks down into plastic dust, which can last for up to a thousand years.
^^^^^copy and paste this part^^^^^
Explanation:Plastic is he hardest material to break down with harm to sea life and land life they may be affordable but costly to the environment.
Members of which group easily lose an electron to form a +1 cation?
halogens
alkaline earth metals
noble gases
alkali metal
Answers
Alkali metals
Explanation:
All alkali metals have 1 valence electron, and they want to lose it, because if they do, they will have 8 valence electrons, which will make them inert.
When an atom lose an electron, the atom becomes a cation, because the atom will have one less electrons than protons.
I hope this helps
The alkali metals easily loose an electron and form a +1 cation.
The periodic table is arranged in groups and periods. The elements in the same group have the same number of valence electrons and similar chemical reactivity. This is why groups of elements are also called families of elements.
The group of the periodic table which its members easily loose electrons and form a +1 cation are the alkali metals.
Learn more:https://brainly.com/question/11857448
How many grams are in 9.97 moles of Be(NO3)2?
Use two digits past the decimal for all values.
Answers
Answer:
1,869.97 grams of Be(NO3)2
Explanation:
Be(NO3)2 = Be N2 O6
Be=9.012182g/mole
N2=28.0134g/mole
O6=96g/mole
therefore Be(NO3)2 gives you 187.56g in one mole
so 9.97 moles means there is 9.97 times more
9.97mole Be(NO3)2 * 187.56g Be(NO3)2/1mole Be(NO3)2 = 1,869.97g of Be(NO3)2
Antoine Lavoisier correctly characterize as an element?
Answers
For the solution resulting from dissolved 0.32 g of naphthalene (C10H8) in 25 g of benzene (C6H6) at temperature of 26.1°C, calculate the vapor pressure lowering, the boiling point elevation, and the freezing point depression. The vapor pressure of benzene at the temperature of the experiment is 100 torr. (Kf of benzene = 2.67 °C/m, Kb of benzene = 5.12 °C/m)
Answers
Answer:
See explanation
Explanation:
Number of moles of naphthalene = 0.32g/128.1705 g/mol = 0.0025 moles
Molality = number of moles/ mass of Solvent in kilograms
Molality = 0.0025/0.025 Kg
Morality = 0.1 m
But
∆T= K × i × m
Where ∆T = boiling point elevation
i= number of particles (this is equal to 1 because naphthalene is molecular and not ionic)
m= molality of naphthalene = 0.1 m
K= boiling point elevation constant = 5.12 °C/m
∆T= 5.12 °C/m ×0.1 = 0.512°C
For freezing point depression
∆T= K× i × m
Where ∆T= freezing point depression
i= number of particles (this is equal to 1 because naphthalene is molecular and not ionic)
m= molality of naphthalene = 0.1 m
K= freezing point depression constant = 2.67 °C/m
∆T= 2.67 °C/m ×0.1 = 0.267°C
From Raoult's law;
∆P = XBPA°
Where;
∆P = vapour pressure lowering
XB = mole fraction of solute
PA° = vapour pressure of pure solvent
Number of moles of solvent = mass/molar mass = 25g/ 78 g/mol= 0.3205 moles
Total number of moles = number of moles of solute + number of moles of solvent = 0.0025 moles + 0.3205 moles = 0.323 moles
Mole fraction of solute = 0.0025 moles/0.323 moles = 0.0077
Vapour pressure of benzene = 100 torr
Therefore;
∆P = 0.0077 × 100torr = 0.77 torr
Hence;
∆P = 0.77 torr
As temperature decreases, what happens to particles in a material?
Particles become further apart / expand?
Particles become slippery / liquid?
Particles become closer together / contract?
Particles become sticky / plasma?
Answers
Answer:
Particles become closer together / contract?
Explanation:
as particles cool down in temp, the particles slow down and compress